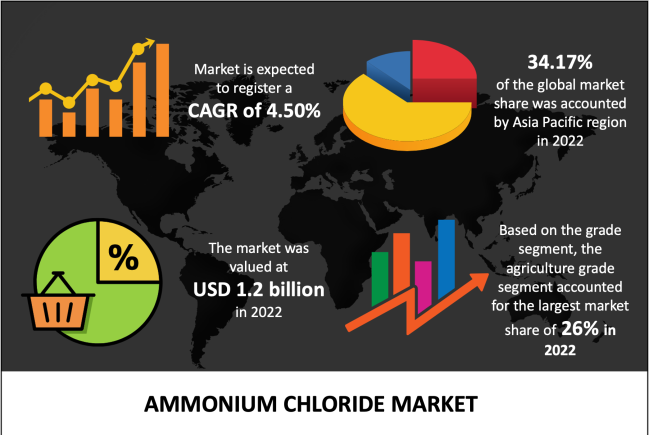
Zinc oxide is an inorganic compound with the formula ZnO. it is insoluble in water and found naturally in the mineral zincite. However, most commercially used zinc oxide is synthetic.
Zinc oxide, also known as Oxozinc, is used in rubbers, plastics, ceramics, glass, lubricants, paints, adhesives, sealants, pigments, cosmetics, etc.
Characterization of Zinc oxide

Zinc oxide is white or yellowish-white in colour, with a large number of uses in different industries. It is insoluble in water but soluble in dilute acids and bases. Some attributes of this substance are:
- Its molar mass is 81.4 g. mol-1.
- Its melting point is 1975 ℃.
- Its boiling point is 2360 ℃.
Applications of Zinc oxide

More than 50% of Zinc oxide use is in the rubber industry. ZnO along with stearic acid is used in the sulfur vulcanization of rubber. ZnO additives also protect the rubber from fungi and UV light.
Zinc oxide also has antibacterial and deodorizing properties. For this reason, it is employed in medical applications such as in baby powder and creams to treat skin irritations. Other uses of Zinc oxide include:
- Paint coatings
- Paint pigments
- Cigarette Filters
- Over-the-counter drug products.
- Nail products
Zinc oxide production
In the French method, metallic zinc is melted in a graphite crucible and vaporized at temperatures above 907 °C. Zinc vapour reacts with the oxygen in the air to give ZnO, accompanied by a drop in its temperature. Then, Zinc oxide particles are transported into a cooling duct and collected in a bag house.
Another method for Zinc oxide production is based on using contaminated zinc composites, such as zinc ores. In this procedure, the zinc precursors are reduced by heating with a source of carbon such as anthracite to produce zinc vapour. Then, it is oxidized as in the French process. In this method, due to the lower purity of the source material, the final product is also of lower quality than the the French method.
Zinc oxide storage
Zinc oxide should be stored in a cool, dry and well-ventilated area. Plus, it should be kept away from incompatible substances such as acids. Other storage conditions include:
- Zinc oxide should be kept in a tightly closed container.
- ZnO should Protect against physical damage.
- Containers of ZnO may be hazardous when empty since they retain product residues.
- The storage temperature of this product is in the range of 15 – 25 °C.
Zinc oxide health hazards
Zinc oxide may cause mechanical irritation to the eyes and respiratory tract. The fume is irritating to the respiratory tract and inhalation of fumes may cause metal fume fever. Some hazardous effects of Zinc oxide are:
- Zinc oxide fume can affect the body if it is inhaled. Exposure of ZnO causes a flu-like illness called metal fume fever. Symptoms include headache, fever, chills, muscle aches, nausea, vomiting, weakness, and tiredness.
- People allergic to ZnO should not use products made of zinc oxide. It may lead to skin irritation, redness of the skin or worsening rashes.
FAQ
What is Zinc oxide?
Zinc Oxide is an inorganic compound which is also known as Zinc White. It has wide applications in rubbers, plastics, ceramics, glass, lubricants and paints.
What are other names for Zinc oxide?
Zinc Oxide is also known as Oxozinc, Zinc White, and Zinc Oxide Powder.
What is Zinc oxide used for?
Zinc Oxide has various applications in different industries. It is used in rubber, textile, cosmetics, Photocatalysis, and pharmaceutical industries.
What are Zinc oxide applications in the plastics industry?
Zinc Oxide is used in the plastics industry to prevent discolouration, maintain heat stability and product transparency.
What is the CAS number of Zinc oxide?
the CAS number of Zinc Oxide is 1314-13-2.
Is Zinc oxide hazardous?
Zinc Oxide is hazardous in case of inhalation. It is also slightly dangerous in case of ingestion. Plus, people allergic to ZnO should not use products made of zinc oxide.