Ethyl Acetate is an organic ester compound with a fruity characteristic odor. It is synthesized mainly via the classic Fischer esterification reaction of ethanol and acetic acid for industrial use. Ethyl Acetate is used as a solvent in making paints, lacquers, varnishes, cleaning liquids, etc.
ArChem provides Ethyl Acetate and other organic solvents with different properties for various industrial applications. for further information please contact our technical sales team in ArChem.

ArChem Ethyl Acetate is a colorless liquid with a characteristic sweet smell. It is the ester of ethanol and acetic acid with the chemical formula of C4H8O2. Ethyl Acetate is commonly used as a solvent, especially for paints, varnishes, lacquers, cleaning mixtures, and perfumes.
This product is highly miscible with all common organic solvents (alcohols, ketones, esters) and only slightly miscibility in water. Its solubility in water is 8.3 g/100 mL (at 20 °C).
ArChem Ethyl acetate is also known as ethyl ethanoate, acetic acid ethyl ester, 1-acetoxyethane. It is generally abbreviated as EtOAC, ETAC, and EA. Chemical and physical properties of ArChem Ethyl Acetate are:
| Molecular Formula | CH3COOC2H5/ C4H8O2 |
| IUPAC name | Ethyl ethanoate |
| Cas Number | 141-78-6 |
| Molecular weight | 88.10 g/mol |
| Density | 0.902 gcm–3 |
| Boiling Point | 77.1 °C |
| Flashpoint | -4 °C |
| Melting Point | −83.6 °C |
| Flammability | Highly flammable |
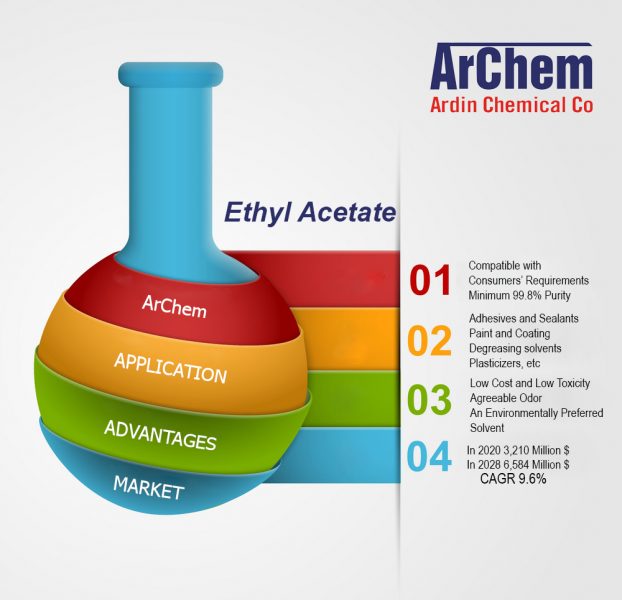
ArChem Ethyl acetate is used as a solvent in different industries. Due to its low cost, low toxicity, and pleasant odor, it is preferred to other solvents. Some of ArChem Ethyl acetate uses include:
Ethyl Acetate is synthesized by the Fischer esterification reaction. In this reaction, ethanol and acetic acid are used as reactants.
CH3CO2H + CH3CH2OH → CH3CO2CH2CH3 + H2O
In industries, it is also produced commercially via the Tishchenko method of condensing two equivalents of acetaldehyde using an alkoxide catalyst. The reaction is given below:
2 CH3CHO → CH3CO2CH2CH3
Storage conditions of Ethyl Acetate are listed below:
Ethyl Acetate is a highly flammable liquid, as well as toxic when inhaled. This chemical also causes irritation when it comes into contact with the eyes or skin. Some of the hazardous effects are listed below:
Personal protective equipment should be worn when handling Ethyl Acetate. Plus, Eye protection must be worn at all times. If contaminated, hands should be washed immediately, and clothing should be removed and replaced.
Ethyl acetate is a chemical solvent, especially for paints, varnishes, lacquers, and cleaning mixtures.
Ethyl ethanoate
Ethyl acetate is toxic to the central nervous system. prolonged exposures can cause irritation and corneal clouding.
It is highly flammable. Heating will cause a rise in pressure with the risk of bursting.
Breathing ethyl acetate can irritate the nose and throat. High exposure can cause unconsciousness.
When handling ethyl acetate, it is recommended that you wear safety glasses, gloves, and a vapor respirator. In the case of inhalation, Seek fresh air immediately. If breathing is absent, seek medical attention immediately.
Due to its low toxicity, ethyl acetate is used as a solvent in inks and paints. Its main function is to dissolve the resin, control the viscosity and modify the drying rate.