Sodium sulfide
Sodium sulfide is a chemical compound with the molecular formula Na2S. This product in pure crystalline form is a colorless solid. It is soluble in water, slightly soluble in alcohol, and insoluble in ether. Sodium sulfide is used in the pulp and paper industry, manufacture of other chemicals, and wastewater treatment.
ArChem provides Sodium sulfide and other raw chemical materials such as MEA, DEA, Soda Ash, and LABSA for various industrial applications. Additionally, ArChem provides customers with other chemical products including Plasticizers, organic solvents, etc. For further information don’t hesitate to contact our technical sales team in ArChem.

What is Sodium sulfide?
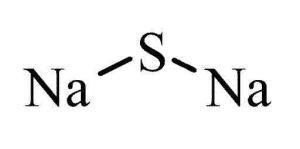
Sodium sulfide (also known as Sodium sulfuret and sodium monosulphide) is a yellow solid flake with a sulfurous smell. It is a sulfide salt, which creates a strong alkaline solution when dissolved in water and can cause chemical burns. Sodium sulfide can react with acids to produce hydrogen sulfide, which is highly toxic.
Sodium sulfide is a toxic and corrosive substance that causes severe skin burns and mucous membrane.
Sodium sulfide technical properties
Na2S is manufactured and sold as a yellow, solid flake with a sulfurous smell. Chemical and physical properties of ArChem Sodium sulfide are:
Molecular Formula | Na2S |
IUPAC name | disodium;sulfide |
Cas Number | 1313-82-2 |
Molecular weight | 78.05 g/mol |
Density | 1.86 gcm–3 |
Boiling Point | 174 °C |
Flashpoint | Non- flammable |
Melting Point | 1176 °C |
Flammability | Flammable |
Sodium sulfide application
Sodium sulfide has different applications in various industries. Some uses of ArChem Sodium sulfide include:
- Pulp and paper industry
- Leather processing
- Manufacture of colors and dyes
- Manufacture of other chemicals
- Waste water treatment
Sodium sulfide production
Sodium sulfide (Na2S) is produced industrially by:
Na2SO4 + 2 C → Na2S + 2 CO2
In the laboratory, Na2S can be produced by the reduction of sulfur with sodium in anhydrous ammonia with a catalytic amount of naphthalene.
2 Na + S → Na2S
Sodium sulfide storage
When using Sodium sulfide, do not eat or drink. Skin cleansing after handling this product is necessary. Storage conditions of ArChem Sodium sulfide are listed below:
- Sodium sulfide should be stored in a cool, dry, and well-ventilated area.
- Sodium sulfide containers should be kept tightly closed.
- Sodium sulfide should be protected against high temperatures, sunlight, and humidity.
- Recommended storage temperature for Sodium sulfide is 15 – 25 °C.
- Sodium sulfide should be kept away from incompatible materials (different metals, aluminum, iron, copper, and zinc)
Sodium sulfide health hazards
Exposure to sodium sulfide can lead to skin irritation, eye irritation, and respiratory tract. Breathing sodium sulfide dusts may aggravate asthma or other pulmonary diseases. Plus, it may cause headaches, dizziness, and vomiting. When handling sodium sulfide, some of the necessary first-aid measures are described below:
- In case of inhalation, remove patient to fresh air. If symptoms persist, seek medical advice.
- In case of ingestion, rinse mouth immediately with water. Call a physician immediately.
- In case of skin contact, wash skin immediately with plenty of water. Call a physician immediately.
- In case of eye contact, flush immediately with plenty of flowing water for 10 to 15 minutes. Call a physician immediately.
Operators should wear appropriate PPE (including gloves, safety glasses, and protective clothing). Plus, they should wash their hands thoroughly after handling
Raw chemical materials are used in the production of chemicals, cosmetics, disinfectants, pharmaceuticals and food industry. ArChem provides customers with the best quality raw chemical materials and competitive prices on the market.Please contact us for a price quote, to request detailed product information and specifications, or to have a sales representative call or visit.
FAQ
What is Sodium sulfide?
Sodium sulfide is a yellow solid flake with a sulfurous smell. It is a sulfide salt, which creates a strong alkaline solution when dissolved in water and can cause chemical burns.
What is Sodium sulfide used for?
Sodium sulfide is used in pulp and paper industry, manufacture of other chemicals, and waste water treatment.
What is the pH of Sodium sulfide?
The ph for Sodium sulfide is 10.4.
What is the price of Sodium sulfide?
ArChem raw chemical materials can be purchased in a variety of volumes. Depending on your orders, prices are different. For more information about Sodium sulfide price, please contact our technical sales team in ArChem.
What is the CAS number of Sodium sulfide?
The CAS number of Sodium sulfide is 1313-82-2.
What is the HS code for Sodium sulfide?
The HS code for Sodium sulfide is 283010
