Ammonium nitrate is a colourless crystalline substance with the chemical formula NH4NO3. It is highly soluble in high temperature water. Ammonium nitrate does not readily burn but will do so if contaminated with combustible materials. This product is commonly used in fertilizers, herbicides, and insecticides. It is also used in explosives for blasting rocks and in mining.
ArChem provides Ammonium nitrate and other raw chemical materials such as Sodium nitrate, Soda Ash, and Ammonium Chloride for various industrial applications. Additionally, ArChem provides customers with other chemical products including Nitrocellulose, Nitrocellulose solution, organic solvents, etc. for further information please contact our technical sales team in ArChem.

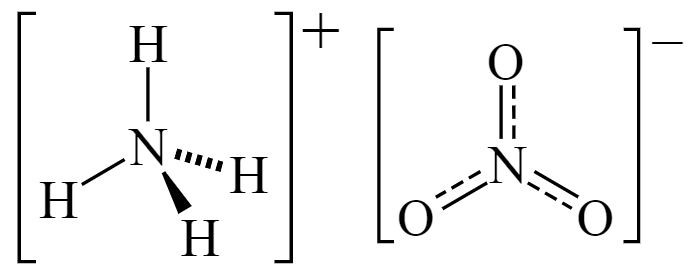
ArChem Ammonium nitrate(also known as Ammonium saltpeter and Ammonium nitricum) is consisting of ions of ammonium and nitrate. The commercial grade contains about 33.5-34 percent nitrogen. Ammonium nitrate is used to make fertilizers and explosives, and as a nutrient in producing antibiotics and yeast.
Pure Ammonium Nitrate does not burn, but it accelerates the burning of combustible materials and produces toxic oxides of nitrogen during combustion. So, it should not be stored near combustible substances. Chemical and physical properties of ArChem Ammonium nitrate are:
| Ammonium nitrate Technical Properties | NH4NO3 |
| IUPAC name | Ammonium nitrate |
| Cas Number | 6484-52-2 |
| Molecular weight | 80.044 g/mol |
| Density | 1.725 gcm–3 |
| Boiling Point | 210 °C |
| Flashpoint | Higher than 93.3 °C |
| Melting Point | 169.6 °C |
| Flammability | Non flammable |

ArChem Ammonium nitrate is used in agriculture as a high-nitrogen fertilizer and is also used as a component of explosive mixtures in mining and civil construction. Some important applications of Ammonium nitrate are listed below:
Ammonium nitrate can be produced by reaction between nitric acid and ammonia:
NH3 + HNO3 → NH4NO3
Another production method is described below:
Ca(NO3)2 + 2 NH3 + CO2 + H2O → 2 NH4NO3 + CaCO3
In this reaction calcium carbonate and ammonium nitrate, may be separately purified or sold combined as calcium ammonium nitrate.
To buy Ammonium nitrate, you should first find Ammonium nitrate suppliers and distributors around the world. Ammonium nitrate suppliers are responsible for procuring high-quality ammonium nitrate from reputable manufacturers. They carefully evaluate the quality, purity, and other characteristics of the chemical to meet the specific requirements of their customers.
Maintaining quality standards is very important in the chemical industry, and Ammonium nitrate suppliers play a crucial role in ensuring the quality of the product they provide. They conduct different quality checks such as testing for purity, nitrogen content, moisture levels, and other specifications, to ensure that the ammonium nitrate meets industry standards.
Plus, distributors of Ammonium nitrate play a critical role in the logistics and distribution of the chemical. They manage transportation, warehousing, and delivery operations. Distributors employ proper handling procedures, optimized storage conditions, and appropriate transportation, to maintain the quality of the chemical throughout the supply chain.
When contacting suppliers or distributors, it’s essential to inquire about their product availability, pricing, minimum order quantities, delivery options, and any other information for your specific needs. ArChem is one of the best suppliers of raw chemical materials at the international level. our partnerships with top suppliers in the market enable us to provide the highest quality ammonium nitrate for different industries.
Storage conditions of ArChem Ammonium nitrate are listed below:
Ammonium nitrate is non-flammable, but it is a strong oxidizing agent that can cause combustible materials (such as wood and paper) to ignite. Some of necessary first-aid measures are described as below:
Operators should wash hands before breaks and after work. they should use personal protective equipment and wear chemical impermeable gloves, when handling Ammonium nitrate to avoid contact with the body, skin, and eyes.
Raw chemical materials are used in the production of chemicals, cosmetics, disinfectants, pharmaceuticals and food additives. ArChem provides customers with the best quality raw chemical materials and competitive prices on the market. Please contact us for a price quote, to request detailed product information and specifications, or to have a sales representative call or visit.
Ammonium nitrate is used to make fertilizers and explosives, and as a nutrient in producing antibiotics and yeast.
Ammonium nitrate is also known as Ammonium saltpeter and Ammonium nitricum.
ArChem raw chemical materials can be purchased in a variety of volumes. Depending on your orders, Prices are different. for more information about Ammonium nitrate price, please contact our technical sales team in ArChem.
The CAS number of Ammonium nitrate is 6484-52-2.
The HS Code of Ammonium nitrate is 31023000.