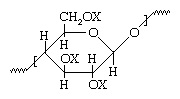
One of the naturally occurring polymers is cellulose which can be made from wood pulp or the short fibers that adhere to cotton seeds. Glucose is a building block with a chemical formula of C6H7O2(OH)3 and has the below molecular structure which is responsible for the formation of cellulose.
The most important raw material of Nitrocellulose or NC, with the chemical formula of [C6H7(NO2)3O5]n and the CAS Number of 9004-70-0, is chemical pulp, i.e. cellulose. It is obtained from cotton.
Unliabove-unalteredose-based products, the degree of substitution is determined indirectly through the nitrogen content of NC for nitrocellulose. While nitrocellulose with a nitrogen content above 12.6 is classified as explosives, Nitrocellulose produced for commercial proposes is divided into three different groups based on their nitrogen content.
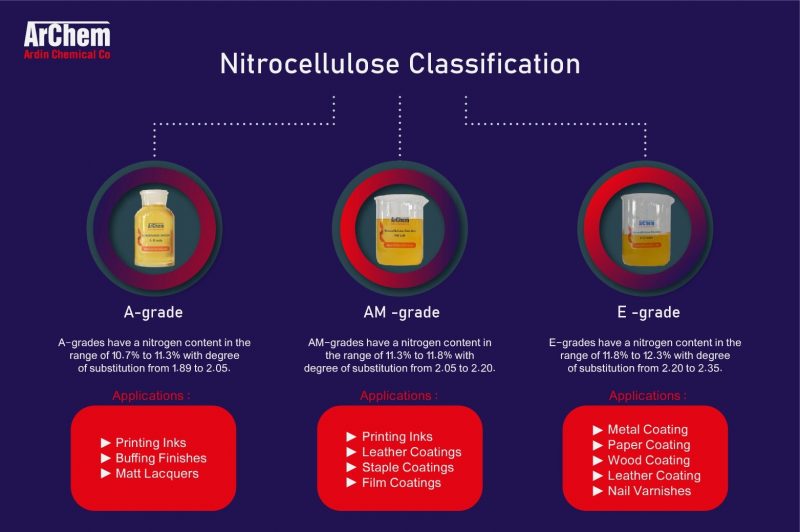
The degree of the substation is coded differently in distinct regions. While nitrocellulose with nitrogen content between 10.7 and 11.3 are known as Grade-A, nitrocellulose with nitrogen content which lies in the range of 11.3 to 11.8 percent are indicated as Grade-AM and nitrocellulose with nitrogen content between 11.8 up to 12.3 percent is known as Grade-E in Europe, nitrocellulose containing the same amount of nitrogen coded as Grades SS, AS, and RS in the USA, respectively.
In addition to this, a table has been prepared and shown bel presenting the degree of substitution of NC code based on different regions.
| Europe | US America/Asia | UK/India/Asia |
| A | SS | M |
| AM | AS | L |
| E | RS | H |
The X shown in the above-unaltered cellulose molecular structure represents a hydrogen (H) atom. This means there are three hydroxyl groups presented iToular formula, resulting in the formation of strong hydrogen bonds between cellulose molecules. These strong hydrogen bonds, prevent the molecule to undergo being smoothened by heat or dissolving by solvent unless they go through chemical decomposition first.
Hydroxyl groups (OH) get replaced by nitro groups (NO2) when cellulose is treated with nitric acid in the presence of a catalyst and water. Chemically the reaction itself is esterification which is an equilibrium reaction.
Although in theory, all hydroxyl groups can be replaced by nitro groups and result in cellulose trinitrate which would contain more than 14% nitrogen, in practice most compound is dinitrates, averaging 1.8 to 2.8 nitro groups per molecule which it would give a containment of 10.5 to 13.5 percent of nitrogen. Since the degree of nitration increases the solubility and flammability of the final product, nitrocellulose containing more than 12.5% nitrogen which is recognized as explosives, will dry to a fluffy white substance known as guncotton.
In order to produce nitrocellulose for commercial proposes, cellulose sheet, and nitrating acids would be added and mixed into a reacting vessel, where the nitration process takes place until the acid is centrifuged from the nitrated product.
After this, the remaining acid would be separated by washing the nitrocellulose slurry in water and boiling it in a caustic solution. Finally, in order to reduce the degradation of the product under exposure to light and heat, various stabilizers would be added to the nitrocellulose product.
Nitrocellulose is characterized based on the type of phlegmatizer added to the NC product and its content, nitrogen content (degree of substitution), and the viscosity (molecular weight) of the final product.
Nitrocellulose is characterized by:
Nitrocellulose is divided into two grades based on the Nitrogen content:
Since industrial nitrocellulose is required by law to contain at least 25% damping agent (alcohol, water) or 18% plasticizer to phlegmatize the nitrocellulose, nitrocellulose finished products are available for commercial proposes as damped NC or granular NC. The only noticeable difference between damped NC or granular NC within the mean viscosity indicated is their physical form and the method of handling them.
Furthermore, NC products are divided into three different groups known as grades A, AM, and E, based on their nitrogen contents and the degree of substitution. NC products from A-grades have a nitrogen content in the range of 10.7% to 11.3% with the degree of substitution from 1.89 to 2.05. Nitrocellulose with Nitrogen content between 11.3% and 11.8% and the degree of substitution of 2.05 to 2.20 belongs to grade AM. E-grade consists of nitrocellulose with a higher nitrogen content than 11.8% up to 12.3% with a degree of substitution between 2.20 and 2.35. NC with a nitrogen content above 12.6 is classified as, explosive.
In addition to these, viscosity as a physical property is another major factor that highly affects the feature of products. Each one of the mentioned grades includes different NC product models whose viscosity is the only variable factor that discriminate them from each other.
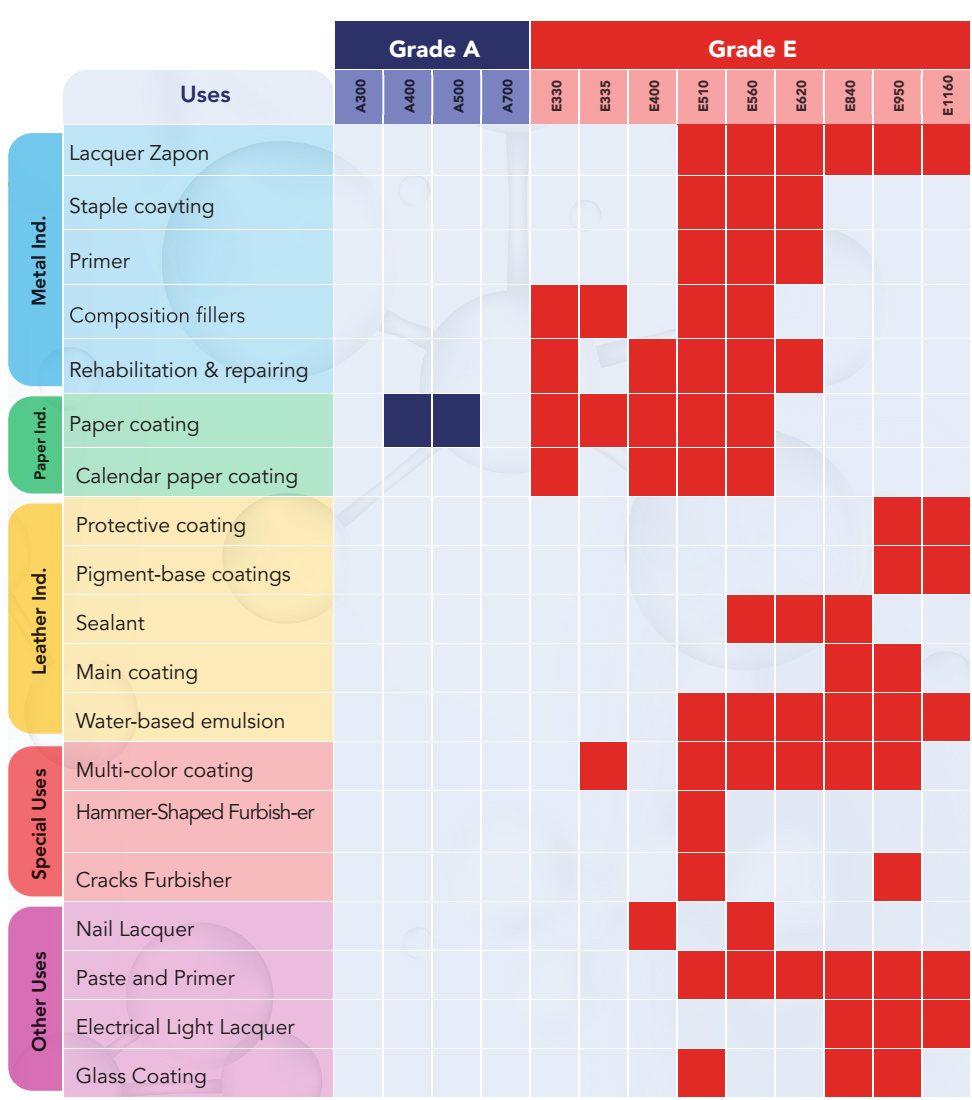
When these physical and chemical factors come together, they can specify the best application for each one of the NC product models. For instance, E-grade nitrocellulose products are normally used for leather coating applications. There is a different types of coatings that are added to leather for different purposes such as protective coatings or base coatings. NC products from E-grade with product model numbers E950 and E1160 are two NC products whose physical and chemical properties make them suitable to be used in protective coating and pigment base coats for leather materials.
Nitrocellulose is widely used as a key component in the production of printing inks and paints, wood finishes, leather finishes, foil and film lacquers, cosmetics, and auto-refinish paints.
ArChem produces industrial Nitrocellulose with a nitrogen content of between 10.7 % and 12.3 %. This is used as a binder in printing inks and coatings.
ArChem company is pleased to supply the highest quality of products from the paint and coating industry as a main chemical raw material in paints and coating industries adjusted to the customer’s needs and expectations. Continual investment in research and development, production equipment, products, and processes put ArChem among the most modern facilities in the middle east. This investment assures a reliable supply of products with consistently high quality.
Due to the usage of one of the most modern production lines and the best raw materials, under the supervision of an experienced and skilled team, our products have one of the highest quality among paint and coating industry products, with highly competitive prices.