Sodium lauryl ether sulfate (SLES), belongs to the group of alkyl ether sulfates. Due to its strong detergency, excellent emulsification, and foamability, SLES is mostly used in hygiene products and cosmetics.
SLES easily dissolves in water, compatible with many surfactants, and is stable in hard water. It is biodegradable with low irritation to the skin and eyes. SLES is produced by ethoxylation of dodecyl alcohol, which is prepared industrially from palm kernel oil or coconut oil.
Characterization of SLES
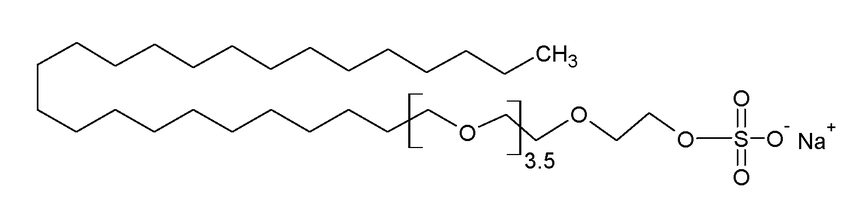
SLES is a clear, light yellow, viscous fluid. It is one of the most important anionic surfactants, salts of fatty alcohol sulfates. The chemical formula of SLES is CH3(CH2)10CH2(OCH2CH2) nOSO3Na.
Sodium lauryl ether sulfate, also known as Sodium laureth sulphate, is used in cosmetic products as a cleansing agent, emulsifier, stabilizer, and solubilizer. Advantages of SLES include:
- Excellent detergency
- Good emulsification property
- High foamability
- Hard water resistant
- Good solubility
- Wide compatibility
- Biodegradable chemical
- Low irritation to skin and eyes
Some properties of SLES include:
- Its molar mass is 3 g. mol-1.
- Its melting point is 206 °C.
- Its boiling point is 4 °C.
- Its density is 01 gcm–3
Applications of SLES
SLES is a surfactant that lowers the surface tension between ingredients. Besides its surface-active characteristics, it possesses emulsifying, cleansing, and foaming properties. Sodium lauryl ether sulfate is used in personal care and home care products, including:
- Hand wash
- Shampoo
- Dish wash
- Liquid detergent
- Toothpaste
- Body wash
plus, it is used as a lubricant, foaming agent, degreasing agent, and dyeing agent in the textile, printing, and leather industries.
SLES Production
The chemical reaction of Lauryl alcohol with petroleum or oil like palm oil and coconut oil produces Sodium Lauryl Sulfate (SLS). Then, in order to obtain SLES, SLS can be introduced to ethylene oxide. This process is called ethoxylation.
Ethylene oxide is a colourless gas that has a sweet odour. It is generally used to produce other chemicals like antifreeze and SLES.
SLES Storage
SLES should be kept in a cool, dry and well-ventilated place. Other storage conditions include:
- SLES should be stored out of direct sunlight.
- Containers of SLES should be tightly closed and sealed after use to prevent leakage.
- SLES should be kept away from heat and sources of ignition.
- SLES should be kept away from incompatible materials.
SLES Health Hazards
SLES is a combustible chemical. It may burn when ignited. Plus, it can easily get ignited through open flames. It reacts with oxidizing agents and strong acids.
SLES is a very toxic chemical. It is hazardous to the human body. It can irritate eyes, skin, and lungs, especially with long-term use.
When handling SLES, personal protective clothing and equipment should be worn as required. Put on protective gloves to prevent skin contact, impervious gloves are necessary.
Additionally, eye protection should be worn while handling SLES to prevent eye contact. Face shields, and chemical goggles, are recommended.
FAQ
What are the benefits of SLES?
SLES has good solvency, favourable hard-water resistance, and high biodegradation. Keep in mind, due to its anionic character, it should not be used with cationic components like cationic surfactants.
What is the most important application of SLES?
SLES is commonly used in cleaning and skincare products such as shampoos and liquid detergents.
What are the disadvantages of SLES?
SLES can irritate eyes, skin, and lungs, especially with long-term use.
Is SLES toxic?
SLES is a very toxic chemical. It is hazardous to the human body. It can lead to serious eye damage if it comes in contact with the eyes. Plus, it is harmful when swallowed. It can cause stomach pain.
How much does SLES cost per kg?
ArChem SLES can be purchased in a variety of volumes. Depending on your orders, Prices are different. for more information about SLES price, please contact our technical sales team in ArChem.
Why SLES is better than SLS?
SLES is much gentler, leaving skin feeling soft, smooth and nourished. Sodium Lauryl Sulfate (SLS) is actually the parent chemical that is modified to make Sodium Laureth Sulfate (SLES).
Why SLES is used in detergent?
SLES improves cleaning power, controls foam, and is cost-effective. Plus, it helps to maintain the stability of liquid detergents.
Is SLES 70% Sodium Lauryl Ether Sulfate?
Sodium Lauryl Ether Sulfate 70%, also known as Sodium Laureth Sulfate, is abbreviated as SLES 70%. It is an anionic surfactant that is used in hygiene products and cosmetics.
